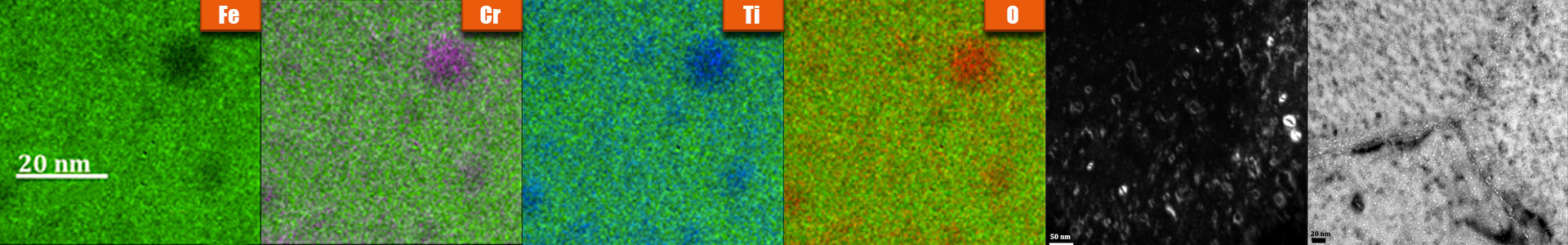
Description
Contacts : Veronika Zinovyeva and Jérôme Roques (Vladimir Sladkov is now working in the Health pole)
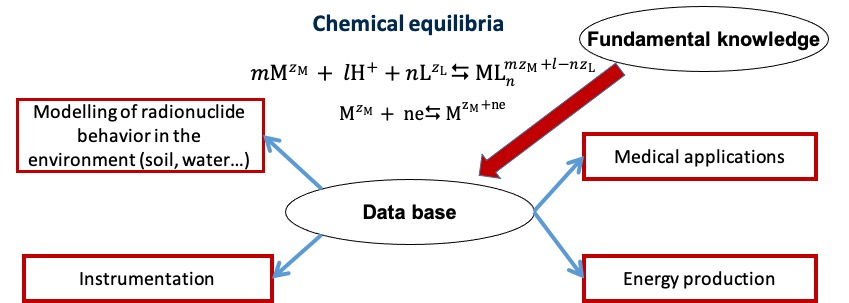
This research topic of the CHIMèNE team is focused on studies of chemical equilibria (hydrolysis, complexation, redox…) of f- and d- elements in aqueous and ionic liquid media. The thermodynamic and kinetic data on these equilibria are important not only for fundamental knowledge, but they have also practical applications for radionuclide environmental monitoring, in medical field and for electrical energy production.
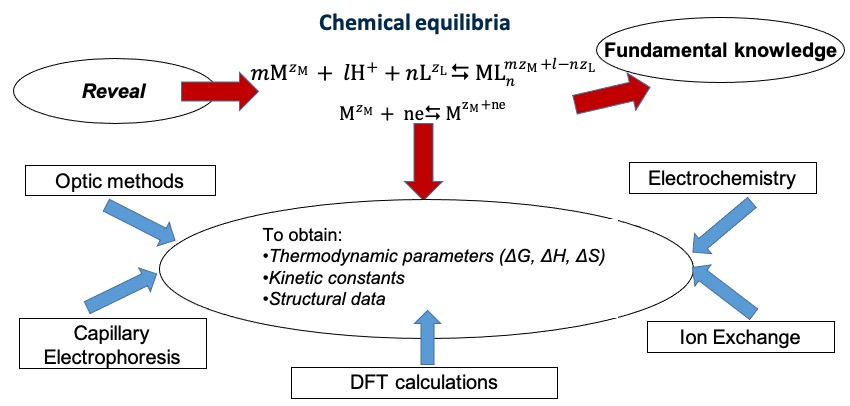
The number of physicochemical experimental methods (spectrophotometry, capillary electrophoresis, electrochemistry…) is successfully used for revealing and characterizing of chemical equilibria (Figure 1). To confirm the experimental data and to obtain the information on the structural parameters and on the chemical bond nature of the complex species, numerical simulations are performed using the density functional theory (DFT) approach.
Collaborations
Health physics pole at IJCLab, University of Burgundy, University of Strasbourg, IRSN, CEA, Triskem
Recent article
● V. Sladkov, J. Roques, M. Meyer. Assignment of complex species by affinity capillary electrophoresis: The case of Th(IV)‐desferrioxamine B. Electrophoresis 41 (2020) 1870-1877